Category:Transition states: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
A '''transition state''' (TS) in chemistry refers to a high-energy, short-lived configuration that occurs during a chemical reaction as reactants transform into products{{Cite|tst:web}}. At this point, the reaction's progress is maximized. In other words, the transition state is a metastable structure that corresponds to a saddle point on the high-dimensional potential energy surface (PES){{Cite|hratchian:schlegel:2005}}. | A '''transition state''' (TS) in chemistry refers to a high-energy, short-lived configuration that occurs during a chemical reaction as reactants transform into products{{Cite|tst:web}}. At this point, the reaction's progress is maximized. In other words, the transition state is a metastable structure that corresponds to a saddle point on the high-dimensional potential energy surface (PES){{Cite|hratchian:schlegel:2005}}. | ||
Identifying the transition state is essential for understanding reaction mechanisms, energy barriers, and rates of reaction. The energy barrier is calculated from the energy difference between the reactant and TS (for the forward reaction) or the product and TS (for the reverse reaction). To the simple energy barrier, additional thermodynamic corrections can be made, including the zero-point vibrational, the population of the vibrational states, and entropy to enable better comparison to the experiment{{Cite|mcquarrie:2000}}. It is also possible to model solid-solid phase transitions in a similar manner{{Cite|henkelman:jcp:2012}} . | Identifying the transition state is essential for understanding reaction mechanisms, energy barriers, and rates of reaction. The energy barrier is calculated from the energy difference between the reactant and TS (for the forward reaction) or the product and TS (for the reverse reaction). To the simple energy barrier, additional thermodynamic corrections can be made, including the zero-point vibrational, the population of the vibrational states, and entropy to enable better comparison to the experiment{{Cite|mcquarrie:2000}}. It is also possible to model solid-solid phase transitions in a similar manner{{Cite|henkelman:jcp:2012}}. | ||
There are many thorough review articles and books on transition state theory (TST){{Cite|truhlar:jpc:1996}}{{Cite|roussel:2023}} and its subsequent development variation TST (VTST){{Cite|truhlar:csr:2017}} which we refer to for the interested reader. There are various methods available to pinpoint transition states in VASP. | There are many thorough review articles and books on transition state theory (TST){{Cite|truhlar:jpc:1996}}{{Cite|roussel:2023}} and its subsequent development variation TST (VTST){{Cite|truhlar:csr:2017}} which we refer to for the interested reader. There are various methods available to pinpoint transition states in VASP. | ||
Revision as of 08:35, 29 October 2024
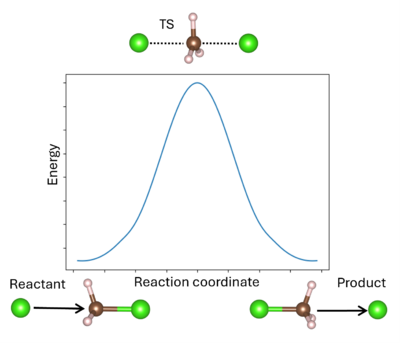
A transition state (TS) in chemistry refers to a high-energy, short-lived configuration that occurs during a chemical reaction as reactants transform into products[1]. At this point, the reaction's progress is maximized. In other words, the transition state is a metastable structure that corresponds to a saddle point on the high-dimensional potential energy surface (PES)[2].
Identifying the transition state is essential for understanding reaction mechanisms, energy barriers, and rates of reaction. The energy barrier is calculated from the energy difference between the reactant and TS (for the forward reaction) or the product and TS (for the reverse reaction). To the simple energy barrier, additional thermodynamic corrections can be made, including the zero-point vibrational, the population of the vibrational states, and entropy to enable better comparison to the experiment[3]. It is also possible to model solid-solid phase transitions in a similar manner[4].
There are many thorough review articles and books on transition state theory (TST)[5][6] and its subsequent development variation TST (VTST)[7] which we refer to for the interested reader. There are various methods available to pinpoint transition states in VASP.
Static methods
There are three methods implemented in VASP for finding transition states (TS) using the static, or harmonic, approach: the improved dimer method (IDM), nudged elastic band (NEB), and following the intrinsic reaction coordinate (IRC). IDM takes a guess structure for the TS and then relaxes it along a trial unstable vibrational mode to a first-order saddle point, i.e. the TS. NEB takes the reactant and product structures and interpolates structures between the two to model the reaction pathway. IRC starts at the TS and follows the TS' unstable vibrational mode to reactant and product.
Improved dimer method
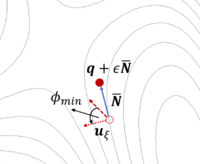
The dimer method[8] is a technique for determining activated transitions without knowledge of the final state. Beginning with a trial transition state (TS) structure, a relaxed TS is obtained. In VASP, the improved dimer method (IDM) by Heyden et al. is implemented. The modification reduces the number of gradient calculations per cycle, improving algorithm performance. A detailed presentation of the method can be found in their paper[9]. The main tag is IBRION=44.
Follow the how-to in order to learn how to perform an IDM calculation.
Nudged elastic bands
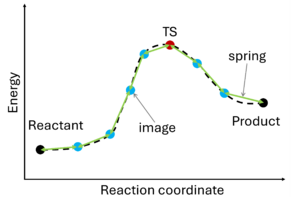
The nudged elastic band (NEB) method[10][11] involves the creation of an initial path connecting the system's initial and final states, utilizing a set of intermediate configurations (IMAGES). Hence, as a prerequisite, the initial and final state (e.g., reactant and product) must be known. The images are interconnected by springs (SPRING), forming a flexible band. Through iterative adjustments (nudging), the positions of the images along the band are modified, minimizing energy until a minimum energy pathway, referred to as the nudged elastic band, is attained.
Learn how to perform an NEB calculation.
Intrinsic reaction coordinate
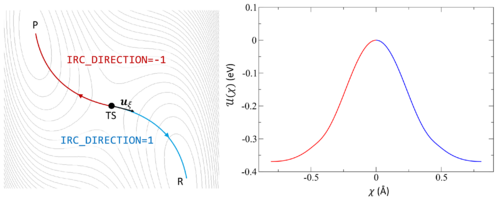
Following the intrinsic reaction coordinate (IRC) implies following the steepest descent path from the transition state to reactants and products. The IRC method employs a classical trajectory integration method with fixed velocity, i.e., the damped-velocity Verlet algorithm, incorporating an adaptive time step such that the dynamic reaction pathway resembles the IRC. Hence, as a prerequisite, the transition state must be known. The main tag is IBRION=40.
Learn how to perform an IRC calculation.
Dynamic methods
An alternative to the static approach for modeling the TS, is to use a dynamic (or anharmonic) approach, that is to use molecular dynamics (MD). The static approach models a single structure for the reactant R, TS, and product P. An MD simulation treats each of these states as an ensemble, a combination of many possible structures.
There is difficulty in trying to find uncommon states or rare events, such as the TS, using MD. An MD simulation is for a short period of time, typically only femto or picoseconds, while seconds would be required to encounter a TS. However, using a blue moon simulation or metadynamics, finding even rare events like the TS becomes feasible.
Blue moon simulations
In constrained molecular dynamics, biased statistical ensembles are generated. One such ensemble is the blue moon average, which connects constrained and unconstrained MD. It shows that the average for a quantity, e.g. a free energy of activation, may be obtained from an ensemble average. Several points can be chosen to perform a constrained MD, along a reaction coordinate defined by ICONST, enabling the entire reaction profile to be modeled from reactants to transition state to products. By restricting the ensemble to lying along the reaction pathway, the transition state is modeled, even though it is a rare event.
References
- ↑ Transition state theory, www.wikipedia.org (2024)
- ↑ H. Hratchian, H. Schlegel, Theory and Application of Computational Chemistry, Chapter 10 - Finding minima, transition states, and following reaction pathways on ab initio potential energy surfaces (2005), p. 195-249
- ↑ D. McQuarrie, Statistical Mechanics, (2000).
- ↑ D. Sheppard, P. Xiao, W. Chemelweski, D. Johnson, G. Henkelman, A generalized solid-state nudged elastic band method, J. Chem. Phys. 136, 074103 (2012).
- ↑ D. Truhlar, B. Garrett, and S. Klippenstein, Current Status of Transition-State Theory, J. Phys. Chem. 100, 12771 (1996).
- ↑ M. Roussel, Foundations of Chemical Kinetics, Chapter 7 - Transition-state theory, (2023), p. 195-249.
- ↑ J. Bao and D. Truhlar, Variational transition state theory: theoretical framework and recent developments, Chem. Soc. Rev. 46, 7548 (2017).
- ↑ G. Henkelman and H. Jónsson, A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives, J. Chem. Phys. 111, 7010–7022 (1999).
- ↑ A. Heyden, A. T. Bell, and F. J. Keil, Efficient methods for finding transition states in chemical reactions: Comparison of improved dimer method and partitioned rational function optimization method, J. Chem. Phys. 123, 224101 (2005).
- ↑ G. Mills, H. Jonsson and G. K. Schenter, Reversible work transition state theory: application to dissociative adsorption of hydrogen, Surf. Sci., 324, 305 (1995).
- ↑ H. Jonsson, G. Mills and K. W. Jacobsen, Nudged Elastic Band Method for Finding Minimum Energy Paths of Transitions, in Classical and Quantum Dynamics in Condensed Phase Simulations, ed. B. J. Berne, G. Ciccotti and D. F. Coker (World Scientific, 1998).
Pages in category "Transition states"
The following 17 pages are in this category, out of 17 total.
